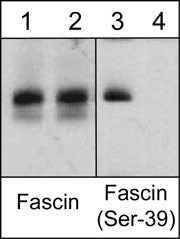
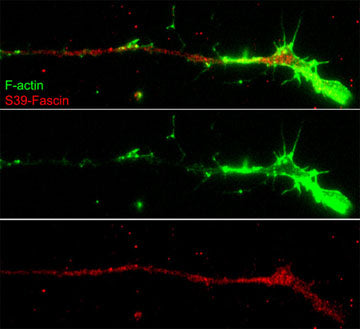
Anti-Fascin (Ser-39), Phosphospecific Antibody
- 产品详情
- 实验流程
- 背景知识
Application
| WB, ICC |
|---|---|
| Primary Accession | Q16658 |
| Host | Rabbit |
| Clonality | Rabbit Polyclonal |
| Isotype | IgG |
| Calculated MW | 54530 Da |
| Gene ID | 6624 |
|---|---|
| Other Names | p55 |
| Target/Specificity | Fascin is an actin filament bundling protein localized to lamellipodia and filopodia where it has important roles in cell motility. Regulation of fascin occurs through PKC-mediated phosphorylation of Ser-39 in the F-actin binding site. Cell permeant peptides that block PKC phosphorylation of Ser-39 increase cell migration, while peptides that block fascin binding to F-actin alter lamellipodial morphology and cause aberrant cell motility. Studies using RNA interference of fascin show that fibroblasts have reduced number and abnormal morphology of filopodia, while Ser-39 phosphorylation status may determine filopodial frequency. In Drosophila neurons, fascin deficiency causes alterations in actin filaments and leads to abnormal morphology of developing neurons. Thus, fascin is a critical element of actin-based motility in various cell types. |
| Dilution | WB~~1:1000 ICC~~N/A |
| Storage | Maintain refrigerated at 2-8°C for up to 6 months. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | Anti-Fascin (Ser-39), Phosphospecific Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Shipping | Blue Ice |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Fascin is an actin filament bundling protein localized to lamellipodia and filopodia where it has important roles in cell motility. Regulation of fascin occurs through PKC-mediated phosphorylation of Ser-39 in the F-actin binding site. Cell permeant peptides that block PKC phosphorylation of Ser-39 increase cell migration, while peptides that block fascin binding to F-actin alter lamellipodial morphology and cause aberrant cell motility. Studies using RNA interference of fascin show that fibroblasts have reduced number and abnormal morphology of filopodia, while Ser-39 phosphorylation status may determine filopodial frequency. In Drosophila neurons, fascin deficiency causes alterations in actin filaments and leads to abnormal morphology of developing neurons. Thus, fascin is a critical element of actin-based motility in various cell types.
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。