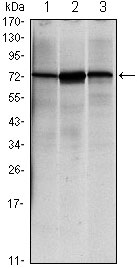
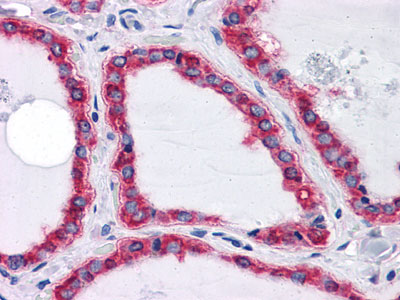
HSPA5 Antibody
Purified Mouse Monoclonal Antibody
- 产品详情
- 实验流程
Application
| WB, IHC, E |
|---|---|
| Primary Accession | P11021 |
| Reactivity | Human |
| Host | Mouse |
| Clonality | Monoclonal |
| Clone Names | 4E3 |
| Isotype | IgG1 |
| Calculated MW | 72333 Da |
| Description | When Chinese hamster K12 cells are starved of glucose, the synthesis of several proteins, called glucose-regulated proteins (GRPs), is markedly increased. Hendershot et al. (1994) (PubMed 8020977) pointed out that one of these, GRP78 (HSPA5), also referred to as 'immunoglobulin heavy chain-binding protein' (BiP), is a member of the heat-shock protein-70 (HSP70) family and is involved in the folding and assembly of proteins in the endoplasmic reticulum (ER). Because so many ER proteins interact transiently with GRP78, it may play a key role in monitoring protein transport through the cell.Probably plays a role in facilitating the assembly of multimeric protein complexes inside the ER.The HSP70 proteins are ubiquitous molecular chaparones that are found in all organisms and tissue types. Like other members of the HSP70 family, BiP is a peptide-binding ATPase that is able to differentiate native proteins from unfolded polypeptides. BiP does not bind to fully folded and assembled proteins, except in the presence of other co-chaparones. BiP is involved in a number of key mechanisms and pathways including polypeptide translocation across the endoplasmic reticulum, folding, assembly, transport of secreted or membrane proteins, and the regulation of calcium homeostasis. Although BiP is relatively abundant, marked increases in BiP occur where there is an accumulation of unfolded polypeptides. For this reason, BiP has been identified as a marker for various disease states that are associated with secretory and transmembrane protein misfolding. |
| Immunogen | Purified recombinant fragment of human HSPA5 expressed in E. Coli. |
| Formulation | Ascitic fluid containing 0.03% sodium azide. |
| Gene ID | 3309 |
|---|---|
| Other Names | 78 kDa glucose-regulated protein, GRP-78, Endoplasmic reticulum lumenal Ca(2+)-binding protein grp78, Heat shock 70 kDa protein 5, Immunoglobulin heavy chain-binding protein, BiP, HSPA5, GRP78 |
| Dilution | WB~~1/500 - 1/2000 IHC~~1/200 - 1/1000 E~~N/A |
| Storage | Maintain refrigerated at 2-8°C for up to 6 months. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | HSPA5 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | HSPA5 (HGNC:5238) |
|---|---|
| Function | Endoplasmic reticulum chaperone that plays a key role in protein folding and quality control in the endoplasmic reticulum lumen (PubMed:2294010, PubMed:23769672, PubMed:23990668, PubMed:28332555). Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10/ERdj5, probably to facilitate the release of DNAJC10/ERdj5 from its substrate (By similarity). Acts as a key repressor of the EIF2AK3/PERK and ERN1/IRE1- mediated unfolded protein response (UPR) (PubMed:11907036, PubMed:1550958, PubMed:19538957, PubMed:36739529). In the unstressed endoplasmic reticulum, recruited by DNAJB9/ERdj4 to the luminal region of ERN1/IRE1, leading to disrupt the dimerization of ERN1/IRE1, thereby inactivating ERN1/IRE1 (By similarity). Also binds and inactivates EIF2AK3/PERK in unstressed cells (PubMed:11907036). Accumulation of misfolded protein in the endoplasmic reticulum causes release of HSPA5/BiP from ERN1/IRE1 and EIF2AK3/PERK, allowing their homodimerization and subsequent activation (PubMed:11907036). Plays an auxiliary role in post-translational transport of small presecretory proteins across endoplasmic reticulum (ER). May function as an allosteric modulator for SEC61 channel-forming translocon complex, likely cooperating with SEC62 to enable the productive insertion of these precursors into SEC61 channel. Appears to specifically regulate translocation of precursors having inhibitory residues in their mature region that weaken channel gating. May also play a role in apoptosis and cell proliferation (PubMed:26045166). |
| Cellular Location | Endoplasmic reticulum lumen. Melanosome. Cytoplasm {ECO:0000250|UniProtKB:P20029}. Cell surface Note=Identified by mass spectrometry in melanosome fractions from stage I to stage IV (PubMed:12643545). Localizes to the cell surface of epithelial cells in response to high levels of free iron (PubMed:20484814, PubMed:24355926, PubMed:27159390) |
Research Areas
For Research Use Only. Not For Use In Diagnostic Procedures.
Application Protocols
Provided below are standard protocols that you may find useful for product applications.
REFERENCES
1. Int J Cancer. 2010 Apr 1;126(7):1562-9. 2. J Virol. 2009 Dec;83(23):12622-5. 3. Mod Pathol. 2010 Jan;23(1):45-53.
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。