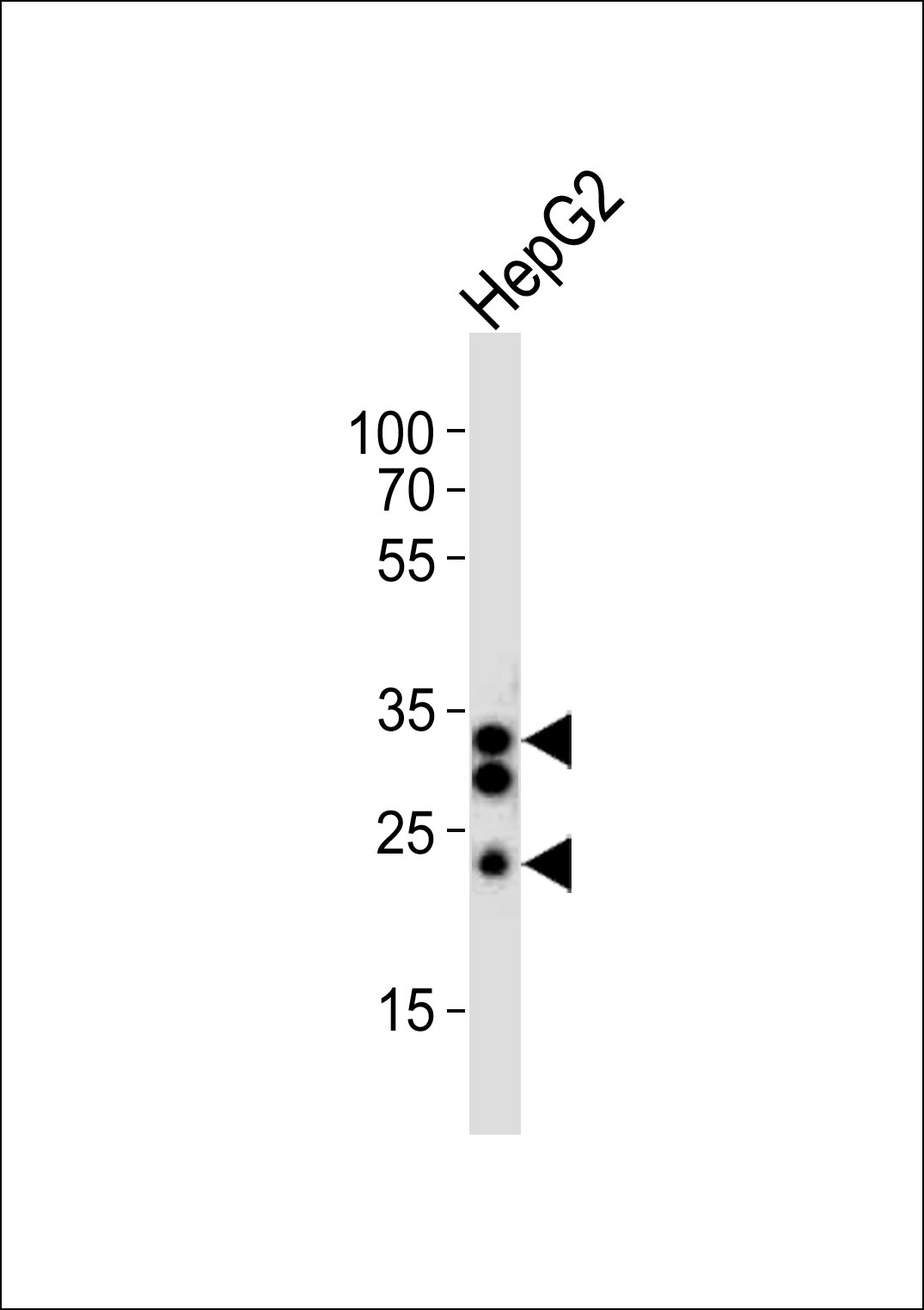
SULT1A1 Antibody (C-term)
Purified Rabbit Polyclonal Antibody (Pab)
- 产品详情
- 实验流程
- 背景知识
Application
| WB, E |
|---|---|
| Primary Accession | P50225 |
| Reactivity | Human |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 34165 Da |
| Gene ID | 6817 |
|---|---|
| Other Names | Sulfotransferase 1A1, ST1A1, Aryl sulfotransferase 1, HAST1/HAST2, Phenol sulfotransferase 1, Phenol-sulfating phenol sulfotransferase 1, P-PST 1, ST1A3, Thermostable phenol sulfotransferase, Ts-PST, SULT1A1, STP, STP1 |
| Target/Specificity | This SULT1A1 antibody is generated from a rabbit immunized with a KLH conjugated synthetic peptide between 246-279 amino acids of human SULT1A1. |
| Dilution | WB~~1:1000 E~~Use at an assay dependent concentration. |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein A column, followed by peptide affinity purification. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | SULT1A1 Antibody (C-term) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | SULT1A1 |
|---|---|
| Synonyms | STP, STP1 |
| Function | Sulfotransferase that utilizes 3'-phospho-5'-adenylyl sulfate (PAPS) as sulfonate donor to catalyze the sulfate conjugation of a wide variety of acceptor molecules bearing a hydroxyl or an amine group. Sulfonation increases the water solubility of most compounds, and therefore their renal excretion, but it can also result in bioactivation to form active metabolites. Displays broad substrate specificity for small phenolic compounds. Plays an important role in the sulfonation of endogenous molecules such as steroid hormones (PubMed:12471039, PubMed:16221673, PubMed:21723874, PubMed:22069470, PubMed:7834621). Mediates the sulfate conjugation of a variety of xenobiotics, including the drugs acetaminophen and minoxidil (By similarity). Mediates also the metabolic activation of carcinogenic N- hydroxyarylamines leading to highly reactive intermediates capable of forming DNA adducts, potentially resulting in mutagenesis (PubMed:7834621). May play a role in gut microbiota-host metabolic interaction. O-sulfonates 4-ethylphenol (4-EP), a dietary tyrosine- derived metabolite produced by gut bacteria. The product 4-EPS crosses the blood-brain barrier and may negatively regulate oligodendrocyte maturation and myelination, affecting the functional connectivity of different brain regions associated with the limbic system (PubMed:35165440). Catalyzes the sulfate conjugation of dopamine (PubMed:8093002). Catalyzes the sulfation of T4 (L-thyroxine/3,5,3',5'- tetraiodothyronine), T3 (3,5,3'-triiodothyronine), rT3 (3,3',5'- triiodothyronine) and 3,3'-T2 (3,3'-diiodothyronine), with a substrate preference of 3,3'-T2 > rT3 > T3 > T4 (PubMed:10199779). |
| Cellular Location | Cytoplasm. |
| Tissue Location | Liver, lung, adrenal, brain, platelets and skin. |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Sulfotransferase that utilizes 3'-phospho-5'-adenylyl sulfate (PAPS) as sulfonate donor to catalyze the sulfate conjugation of catecholamines, phenolic drugs and neurotransmitters. Has also estrogen sulfotransferase activity. responsible for the sulfonation and activation of minoxidil. Is Mediates the metabolic activation of carcinogenic N- hydroxyarylamines to DNA binding products and could so participate as modulating factor of cancer risk.
REFERENCES
Zhu X.,et al.Biochem. Biophys. Res. Commun. 195:120-127(1993).
Zhu X.,et al.Biochem. Biophys. Res. Commun. 192:671-676(1993).
Wilborn T.W.,et al.Mol. Pharmacol. 43:70-77(1993).
Yamazoe Y.,et al.Chem. Biol. Interact. 92:107-117(1994).
Hwang S.-R.,et al.Biochem. Biophys. Res. Commun. 207:701-707(1995).
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。