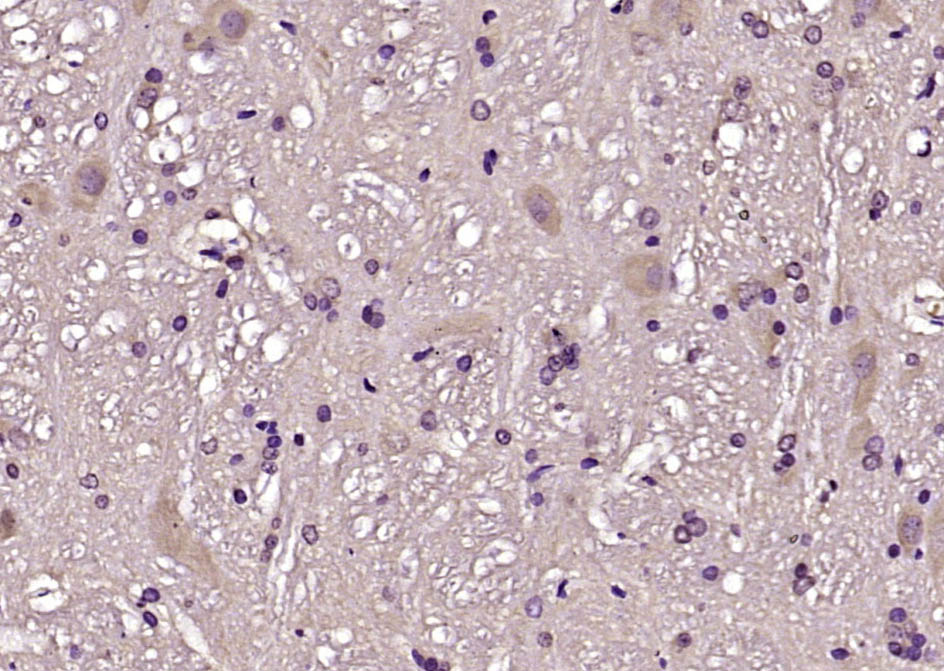
CBLN1 Rabbit pAb
CBLN1 Rabbit pAb
- 产品详情
- 实验流程
- 背景知识
Application
| IHC-P, IHC-F, IF |
|---|---|
| Primary Accession | P23435 |
| Reactivity | Rat |
| Predicted | Human, Mouse, Pig, Rabbit |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 21097 Da |
| Physical State | Liquid |
| Immunogen | KLH conjugated synthetic peptide derived from human CBLN1 |
| Epitope Specificity | 51-150/193 |
| Isotype | IgG |
| Purity | affinity purified by Protein A |
| Buffer | 0.01M TBS (pH7.4) with 1% BSA, 0.02% Proclin300 and 50% Glycerol. |
| SUBCELLULAR LOCATION | Secreted. Membrane. Cell junction, synapse. |
| SIMILARITY | Contains 1 C1q domain. |
| SUBUNIT | Homohexamer; disulfide-linked homotrimers. The trimers are assembled via the globular C1q domains. The trimers associate via N-terminal cysteine residues to form disulfide-linked hexamers. Probably forms a heteomeric complex with CBLN3. May interact with CBLN2 and CBLN4 |
| Post-translational modifications | The proteolytic processing to yield cerebellin seems to occur either prior to the secretion by presynaptic neurons and subsequent oligomerization or in some other location after release of the mature protein. |
| Important Note | This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications. |
| Background Descriptions | Cerebellin (CER), which was originally isolated from rat cerebellum, is a hexadecapeptide derived from a larger precursor Cerebellin 1, also designated precerebellin 1 or Cbln1. Four propeptides, Cerebellin 1, Cerebellin 2 (Cbln2), Cerebellin 3 (Cbln3), and Cerebellin 4 (Cbln4), comprise the precerebellin subfamily within the C1q protein family. Cerebellin family members act as transneuronal regulators of synapse development and synaptic plasticity in various brain regions. CER and it metabolite des-Ser1-cerebellin are also expressed in several extra-cerebellar tissues, including adrenal gland. Cerebellin 1, 2 and 3 assemble into homomeric and heteromeric complexes, thereby influencing each other’s degradation and secretion. Cerebellin 3 is not able to form homomeric complexes, and can only be secreted upon forming a heteromeric complex with Cerebellin 1. Decreased concentrations of CER has been found in the brain of patients with olivopontocerebellar atrophy (OPCA) and Shy-Drager syndrome, suggesting a role for CER in the pathology of these diseases. |
| Gene ID | 869 |
|---|---|
| Other Names | Cerebellin-1, Precerebellin, Cerebellin, CER, [des-Ser1]-cerebellin, CBLN1 |
| Target/Specificity | In the Purkinje cells postsynaptic structures. In the cerebellum, cerebellin is much less abundant than [des-Ser1]-cerebellin. |
| Dilution | IHC-P=1:100-500,IHC-F=1:100-500,IF=1:100-500 |
| Storage | Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. |
| Name | CBLN1 |
|---|---|
| Function | Required for synapse integrity and synaptic plasticity. During cerebellar synapse formation, essential for the matching and maintenance of pre- and post-synaptic elements at parallel fiber- Purkinje cell synapses, the establishment of the proper pattern of climbing fiber-Purkinje cell innervation, and induction of long-term depression at parallel fiber-Purkinje cell synapses. Plays a role as a synaptic organizer that acts bidirectionally on both pre- and post- synaptic components. On the one hand induces accumulation of synaptic vesicles in the pre-synaptic part by binding with NRXN1 and in other hand induces clustering of GRID2 and its associated proteins at the post-synaptic site through association of GRID2. NRXN1-CBLN1-GRID2 complex directly induces parallel fiber protrusions that encapsulate spines of Purkinje cells leading to accumulation of GRID2 and synaptic vesicles. Required for CBLN3 export from the endoplasmic reticulum and secretion (By similarity). NRXN1-CBLN1-GRID2 complex mediates the D- Serine-dependent long term depression signals and AMPA receptor endocytosis (PubMed:27418511). Essential for long-term maintenance but not establishment of excitatory synapses (By similarity). Inhibits the formation and function of inhibitory GABAergic synapses in cerebellar Purkinje cells (By similarity). |
| Cellular Location | Secreted {ECO:0000250|UniProtKB:Q9R171}. Postsynaptic cell membrane {ECO:0000250|UniProtKB:Q9R171} |
| Tissue Location | In the Purkinje cells postsynaptic structures. In the cerebellum, cerebellin is much less abundant than [des-Ser1]- cerebellin |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Cerebellin (CER), which was originally isolated from rat cerebellum, is a hexadecapeptide derived from a larger precursor Cerebellin 1, also designated precerebellin 1 or Cbln1. Four propeptides, Cerebellin 1, Cerebellin 2 (Cbln2), Cerebellin 3 (Cbln3), and Cerebellin 4 (Cbln4), comprise the precerebellin subfamily within the C1q protein family. Cerebellin family members act as transneuronal regulators of synapse development and synaptic plasticity in various brain regions. CER and it metabolite des-Ser1-cerebellin are also expressed in several extra-cerebellar tissues, including adrenal gland. Cerebellin 1, 2 and 3 assemble into homomeric and heteromeric complexes, thereby influencing each other’s degradation and secretion. Cerebellin 3 is not able to form homomeric complexes, and can only be secreted upon forming a heteromeric complex with Cerebellin 1. Decreased concentrations of CER has been found in the brain of patients with olivopontocerebellar atrophy (OPCA) and Shy-Drager syndrome, suggesting a role for CER in the pathology of these diseases.
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。