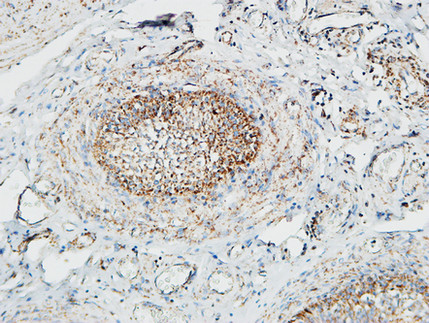
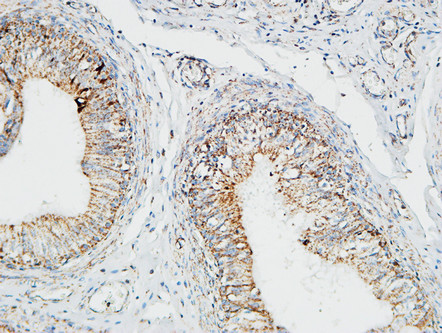
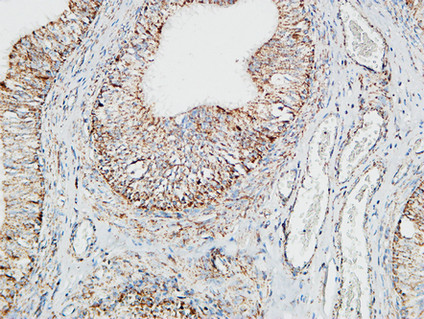
Pin1 Polyclonal Antibody
- 产品详情
- 实验流程
- 背景知识
Application
| IHC-P, IF, ICC, E |
|---|---|
| Primary Accession | Q13526 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 18243 Da |
| Gene ID | 5300 |
|---|---|
| Other Names | PIN1; Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1; Peptidyl-prolyl cis-trans isomerase Pin1; PPIase Pin1; Rotamase Pin1 |
| Dilution | IHC-P~~Immunohistochemistry: 1/100 - 1/300. Immunofluorescence: 1/200 - 1/1000. ELISA: 1/5000. Not yet tested in other applications. IF~~1:50~200 ICC~~N/A E~~N/A |
| Format | Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.09% (W/V) sodium azide. |
| Storage Conditions | -20℃ |
| Name | PIN1 |
|---|---|
| Function | Peptidyl-prolyl cis/trans isomerase (PPIase) that binds to and isomerizes specific phosphorylated Ser/Thr-Pro (pSer/Thr-Pro) motifs (PubMed:21497122, PubMed:23623683, PubMed:29686383). By inducing conformational changes in a subset of phosphorylated proteins, acts as a molecular switch in multiple cellular processes (PubMed:21497122, PubMed:22033920, PubMed:23623683). Displays a preference for acidic residues located N-terminally to the proline bond to be isomerized. Regulates mitosis presumably by interacting with NIMA and attenuating its mitosis-promoting activity. Down-regulates kinase activity of BTK (PubMed:16644721). Can transactivate multiple oncogenes and induce centrosome amplification, chromosome instability and cell transformation. Required for the efficient dephosphorylation and recycling of RAF1 after mitogen activation (PubMed:15664191). Binds and targets PML and BCL6 for degradation in a phosphorylation-dependent manner (PubMed:17828269). Acts as a regulator of JNK cascade by binding to phosphorylated FBXW7, disrupting FBXW7 dimerization and promoting FBXW7 autoubiquitination and degradation: degradation of FBXW7 leads to subsequent stabilization of JUN (PubMed:22608923). May facilitate the ubiquitination and proteasomal degradation of RBBP8/CtIP through CUL3/KLHL15 E3 ubiquitin-protein ligase complex, hence favors DNA double-strand repair through error-prone non-homologous end joining (NHEJ) over error-free, RBBP8-mediated homologous recombination (HR) (PubMed:23623683, PubMed:27561354). Upon IL33-induced lung inflammation, catalyzes cis-trans isomerization of phosphorylated IRAK3/IRAK-M, inducing IRAK3 stabilization, nuclear translocation and expression of pro-inflammatory genes in dendritic cells (PubMed:29686383). Catalyzes cis-trans isomerization of phosphorylated phosphoglycerate kinase PGK1 under hypoxic conditions to promote its binding to the TOM complex and targeting to the mitochondrion (PubMed:26942675). Acts as a negative regulator of adipocyte browning by binding to phosphorylated PRDM16, targeting PRDM16 for degradation (By similarity). |
| Cellular Location | Nucleus. Nucleus speckle. Cytoplasm Note=Colocalizes with NEK6 in the nucleus (PubMed:16476580). Mainly localized in the nucleus but phosphorylation at Ser-71 by DAPK1 results in inhibition of its nuclear localization (PubMed:21497122) |
| Tissue Location | Expressed in immune cells in the lung (at protein level) (PubMed:29686383). The phosphorylated form at Ser-71 is expressed in normal breast tissue cells but not in breast cancer cells |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Peptidyl-prolyl cis/trans isomerase (PPIase) that binds to and isomerizes specific phosphorylated Ser/Thr-Pro (pSer/Thr- Pro) motifs. By inducing conformational changes in a subset of phosphorylated proteins, acts as a molecular switch in multiple cellular processes (PubMed:21497122, PubMed:22033920, Ref. 21). Displays a preference for acidic residues located N-terminally to the proline bond to be isomerized. Regulates mitosis presumably by interacting with NIMA and attenuating its mitosis-promoting activity. Down-regulates kinase activity of BTK (PubMed:16644721). Can transactivate multiple oncogenes and induce centrosome amplification, chromosome instability and cell transformation. Required for the efficient dephosphorylation and recycling of RAF1 after mitogen activation (PubMed:15664191). Binds and targets PML and BCL6 for degradation in a phosphorylation-dependent manner (PubMed:17828269). Acts as a regulator of JNK cascade by binding to phosphorylated FBXW7, disrupting FBXW7 dimerization and promoting FBXW7 autoubiquitination and degradation: degradation of FBXW7 leads to subsequent stabilization of JUN (PubMed:22608923). May facilitate the ubiquitination and proteasomal degradation of RBBP8/CtIP through CUL3/KLHL15 E3 ubiquitin-protein ligase complex, hence favors DNA double-strand repair through error-prone non-homologous end joining (NHEJ) over error-free, RBBP8-mediated homologous recombination (HR) (PubMed:23623683, PubMed:27561354).
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。