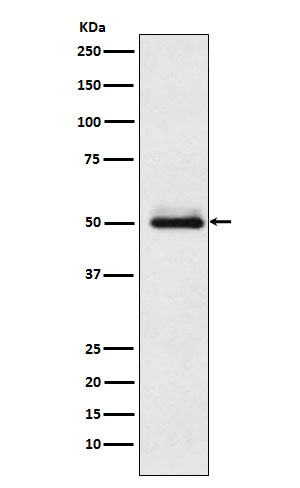
Cytochrome P450 2C9 Rabbit mAb
- 产品详情
- 实验流程
Application
| WB, IF, FC, ICC |
|---|---|
| Primary Accession | P11712 |
| Reactivity | Human |
| Host | Rabbit |
| Clonality | Monoclonal Antibody |
| Isotype | IgG |
| Conjugate | Unconjugated |
| Immunogen | A synthesized peptide derived from human Cytochrome P450 2C9 |
| Purification | Affinity Chromatography |
| Calculated MW | 55628 Da |
| Gene ID | 1559 |
|---|---|
| Other Names | CYP2C9 |
| Dilution | WB~~1/500-1/1000 IF~~1:50~200 FC~~1:10~50 ICC~~N/A |
| Format | Liquid in 10mM PBS, pH 7.4, 150mM sodium chloride, 0.05% BSA, 0.02% sodium azide and 50% glycerol. |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Name | CYP2C9 {ECO:0000303|PubMed:11950794, ECO:0000312|HGNC:HGNC:2623} |
|---|---|
| Function | A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids and steroids (PubMed:12865317, PubMed:15766564, PubMed:19965576, PubMed:21576599, PubMed:7574697, PubMed:9435160, PubMed:9866708). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase) (PubMed:12865317, PubMed:15766564, PubMed:19965576, PubMed:21576599, PubMed:7574697, PubMed:9435160, PubMed:9866708). Catalyzes the epoxidation of double bonds of polyunsaturated fatty acids (PUFA) (PubMed:15766564, PubMed:19965576, PubMed:7574697, PubMed:9866708). Catalyzes the hydroxylation of carbon-hydrogen bonds. Metabolizes cholesterol toward 25-hydroxycholesterol, a physiological regulator of cellular cholesterol homeostasis (PubMed:21576599). Exhibits low catalytic activity for the formation of catechol estrogens from 17beta- estradiol (E2) and estrone (E1), namely 2-hydroxy E1 and E2 (PubMed:12865317). Catalyzes bisallylic hydroxylation and hydroxylation with double-bond migration of polyunsaturated fatty acids (PUFA) (PubMed:9435160, PubMed:9866708). Also metabolizes plant monoterpenes such as limonene. Oxygenates (R)- and (S)-limonene to produce carveol and perillyl alcohol (PubMed:11950794). Contributes to the wide pharmacokinetics variability of the metabolism of drugs such as S- warfarin, diclofenac, phenytoin, tolbutamide and losartan (PubMed:25994031). |
| Cellular Location | Endoplasmic reticulum membrane; Peripheral membrane protein. Microsome membrane; Peripheral membrane protein |
Research Areas
For Research Use Only. Not For Use In Diagnostic Procedures.
Application Protocols
Provided below are standard protocols that you may find useful for product applications.
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.
¥ 1,500.00
Cat# AP77082























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。