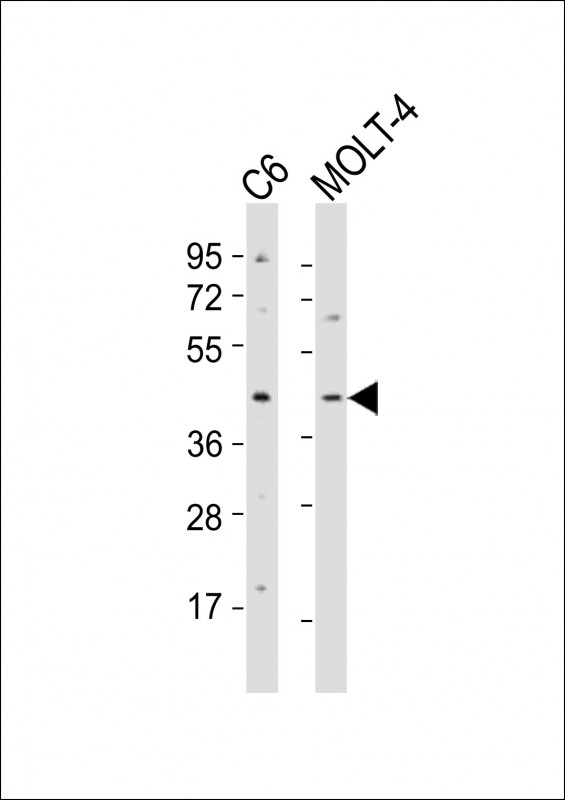
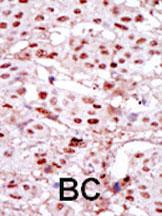
CKII alpha (CSNK2A1) Antibody (Center)
Purified Rabbit Polyclonal Antibody (Pab)
- 产品详情
- 实验流程
- 背景知识
Application
| IHC-P, WB, E |
|---|---|
| Primary Accession | P68400 |
| Other Accession | P28020, P19139, P33674, Q60737, P21868, P68399 |
| Reactivity | Human, Rat, Mouse |
| Predicted | Bovine, Chicken, Rabbit, Rat, Xenopus |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | Rabbit IgG |
| Calculated MW | 45144 Da |
| Antigen Region | 240-269 aa |
| Gene ID | 1457 |
|---|---|
| Other Names | Casein kinase II subunit alpha, CK II alpha, CSNK2A1, CK2A1 |
| Target/Specificity | This CKII alpha (CSNK2A1) antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 240-269 amino acids from the Central region of human CKII alpha (CSNK2A1). |
| Dilution | IHC-P~~1:100~500 WB~~1:2000 E~~Use at an assay dependent concentration. |
| Format | Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. |
| Storage | Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | CKII alpha (CSNK2A1) Antibody (Center) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | CSNK2A1 |
|---|---|
| Synonyms | CK2A1 |
| Function | Catalytic subunit of a constitutively active serine/threonine-protein kinase complex that phosphorylates a large number of substrates containing acidic residues C-terminal to the phosphorylated serine or threonine (PubMed:11239457, PubMed:11704824, PubMed:16193064, PubMed:18411307, PubMed:18583988, PubMed:18678890, PubMed:19188443, PubMed:20545769, PubMed:20625391, PubMed:22017874, PubMed:22406621, PubMed:24962073, PubMed:30898438, PubMed:31439799). Regulates numerous cellular processes, such as cell cycle progression, apoptosis and transcription, as well as viral infection (PubMed:12631575, PubMed:19387551, PubMed:19387552). May act as a regulatory node which integrates and coordinates numerous signals leading to an appropriate cellular response (PubMed:12631575, PubMed:19387551, PubMed:19387552). During mitosis, functions as a component of the p53/TP53-dependent spindle assembly checkpoint (SAC) that maintains cyclin-B-CDK1 activity and G2 arrest in response to spindle damage (PubMed:11704824, PubMed:19188443). Also required for p53/TP53-mediated apoptosis, phosphorylating 'Ser-392' of p53/TP53 following UV irradiation (PubMed:11239457). Phosphorylates a number of DNA repair proteins in response to DNA damage, such as MDC1, MRE11, RAD9A, RAD51 and HTATSF1, promoting their recruitment to DNA damage sites (PubMed:18411307, PubMed:18583988, PubMed:18678890, PubMed:20545769, PubMed:21482717, PubMed:22325354, PubMed:26811421, PubMed:28512243, PubMed:30898438, PubMed:35597237). Can also negatively regulate apoptosis (PubMed:16193064, PubMed:22184066). Phosphorylates the caspases CASP9 and CASP2 and the apoptotic regulator NOL3 (PubMed:16193064). Phosphorylation protects CASP9 from cleavage and activation by CASP8, and inhibits the dimerization of CASP2 and activation of CASP8 (PubMed:16193064). Phosphorylates YY1, protecting YY1 from cleavage by CASP7 during apoptosis (PubMed:22184066). Regulates transcription by direct phosphorylation of RNA polymerases I, II, III and IV (PubMed:12631575, PubMed:19387550, PubMed:19387551, PubMed:19387552, PubMed:23123191). Also phosphorylates and regulates numerous transcription factors including NF-kappa-B, STAT1, CREB1, IRF1, IRF2, ATF1, ATF4, SRF, MAX, JUN, FOS, MYC and MYB (PubMed:12631575, PubMed:19387550, PubMed:19387551, PubMed:19387552, PubMed:23123191). Phosphorylates Hsp90 and its co-chaperones FKBP4 and CDC37, which is essential for chaperone function (PubMed:19387550). Mediates sequential phosphorylation of FNIP1, promoting its gradual interaction with Hsp90, leading to activate both kinase and non-kinase client proteins of Hsp90 (PubMed:30699359). Regulates Wnt signaling by phosphorylating CTNNB1 and the transcription factor LEF1 (PubMed:19387549). Acts as an ectokinase that phosphorylates several extracellular proteins (PubMed:12631575, PubMed:19387550, PubMed:19387551, PubMed:19387552). During viral infection, phosphorylates various proteins involved in the viral life cycles of EBV, HSV, HBV, HCV, HIV, CMV and HPV (PubMed:12631575, PubMed:19387550, PubMed:19387551, PubMed:19387552). Phosphorylates PML at 'Ser-565' and primes it for ubiquitin-mediated degradation (PubMed:20625391, PubMed:22406621). Plays an important role in the circadian clock function by phosphorylating BMAL1 at 'Ser-90' which is pivotal for its interaction with CLOCK and which controls CLOCK nuclear entry (By similarity). Phosphorylates CCAR2 at 'Thr-454' in gastric carcinoma tissue (PubMed:24962073). Phosphorylates FMR1, promoting FMR1-dependent formation of a membraneless compartment (PubMed:30765518, PubMed:31439799). May phosphorylate histone H2A on 'Ser-1' (PubMed:38334665). |
| Cellular Location | Nucleus |
| Tissue Location | Expressed in gastric carcinoma tissue and the expression gradually increases with the progression of the carcinoma (at protein level). |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Casein kinase II is a serine/threonine protein kinase that phosphorylates acidic proteins such as casein. The kinase exists as a tetramer and is composed of an alpha, an alpha-prime, and two beta subunits. The alpha subunits contain the catalytic activity while the beta subunits undergo autophosphorylation.
REFERENCES
Miyata, Y., et al., Mol. Cell. Biol. 24(9):4065-4074 (2004).
Loizou, J.I., et al., Cell 117(1):17-28 (2004).
Filhol, O., et al., EMBO Rep. 5(4):351-355 (2004).
Kulartz, M., et al., Biochem. Biophys. Res. Commun. 315(4):1011-1017 (2004).
Sachs, N.A., et al., J. Neurochem. 88(1):51-62 (2004).
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。