Cytochrome P450 1A2 Antibody
Rabbit mAb
- 产品详情
- 实验流程
Application
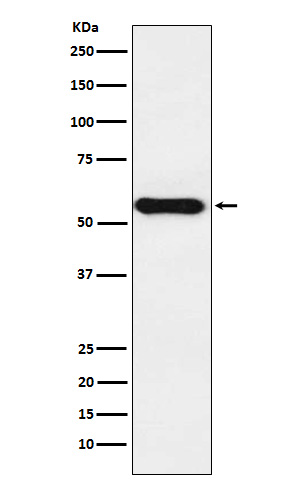
| WB, IF, FC, ICC |
|---|---|
| Primary Accession | P05177 |
| Reactivity | Human |
| Clonality | Monoclonal |
| Other Names | CP12; CYP1A2; CYPIA2; P3 450; P450 4; P450 P3; |
| Isotype | Rabbit IgG |
| Host | Rabbit |
| Calculated MW | 58407 Da |
| Dilution | WB 1:500~1:2000 ICC/IF 1:50~1:200 FC 1:500 |
|---|---|
| Purification | Affinity-chromatography |
| Immunogen | A synthesized peptide derived from human Cytochrome P450 1A2 |
| Description | Cytochromes P450 are a group of heme-thiolate monooxygenases. In liver microsomes, this enzyme is involved in an NADPH-dependent electron transport pathway. |
| Storage Condition and Buffer | Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol. Store at +4°C short term. Store at -20°C long term. Avoid freeze / thaw cycle. |
| Name | CYP1A2 {ECO:0000303|PubMed:2575218, ECO:0000312|HGNC:HGNC:2596} |
|---|---|
| Function | A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins (PubMed:10681376, PubMed:11555828, PubMed:12865317, PubMed:19965576, PubMed:9435160). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase) (PubMed:10681376, PubMed:11555828, PubMed:12865317, PubMed:19965576, PubMed:9435160). Catalyzes the hydroxylation of carbon-hydrogen bonds (PubMed:11555828, PubMed:12865317). Exhibits high catalytic activity for the formation of hydroxyestrogens from estrone (E1) and 17beta- estradiol (E2), namely 2-hydroxy E1 and E2 (PubMed:11555828, PubMed:12865317). Metabolizes cholesterol toward 25-hydroxycholesterol, a physiological regulator of cellular cholesterol homeostasis (PubMed:21576599). May act as a major enzyme for all-trans retinoic acid biosynthesis in the liver. Catalyzes two successive oxidative transformation of all-trans retinol to all-trans retinal and then to the active form all-trans retinoic acid (PubMed:10681376). Primarily catalyzes stereoselective epoxidation of the last double bond of polyunsaturated fatty acids (PUFA), displaying a strong preference for the (R,S) stereoisomer (PubMed:19965576). Catalyzes bisallylic hydroxylation and omega-1 hydroxylation of PUFA (PubMed:9435160). May also participate in eicosanoids metabolism by converting hydroperoxide species into oxo metabolites (lipoxygenase-like reaction, NADPH- independent) (PubMed:21068195). Plays a role in the oxidative metabolism of xenobiotics. Catalyzes the N-hydroxylation of heterocyclic amines and the O-deethylation of phenacetin (PubMed:14725854). Metabolizes caffeine via N3-demethylation (Probable). |
| Cellular Location | Endoplasmic reticulum membrane; Peripheral membrane protein. Microsome membrane; Peripheral membrane protein |
| Tissue Location | Liver. |
Research Areas
For Research Use Only. Not For Use In Diagnostic Procedures.
Application Protocols
Provided below are standard protocols that you may find useful for product applications.
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.
¥ 1,500.00
Cat# AP91933























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。